Background: Watch and wait is standard of care in patients with early stage chronic lymphocytic leukemia (CLL). In the CLL12 trial, 515 stage Binet-A patients were risk stratified and randomized 1:1 to ibrutinib (ibr, n=182) or placebo (pcb, n=181) when assigned to (very) high or intermediate risk, while low risk patients were only observed (w&w, n=152). Treatment with ibr was associated with longer progression-free survival (PFS) (event defined as progression or death) and event-free survival (EFS) (event defined as death, symptomatic progression or initiation of CLL treatment) but not overall survival (OS) when compared to pcb (Langerbeins et al. EHA 2023). However, it is unclear if specific subgroups defined by genetic markers may derive particular benefit from early ibr treatment.
Methods: Genetic markers were analyzed in all 515 patients at study entry. Genomic aberrations were assessed by FISH, IGHV mutational status by sequencing with a threshold of 98% homology and gene mutations via targeted NGS for TP53, FBXW7, NOTCH1, SF3B1, NRAS, KRAS, BRAF, BIRC3, MYD88, NFKBIE, EGR2 and XPO1.
Results: The prevalence of genomic aberrations and gene mutations is shown in the table 1.
At a median follow up of 69.3 months, there were 166 events for EFS and 32 for OS respectively. EFS was shorter with del(17p) (compared to del(13q): Hazard ratio (HR) 2.89, 95%CI 1.14-7.37, p<0.05), del(11q) (HR 4.39, 95%CI 2.48-7.76, p<0.001); +(12) (HR 1.78, 95%CI 1.01-3.13, p<0.05) in the pcb arm, while with ibr only del(17p) (HR 5.07, 95%CI 1.70-15.17 p<0.01) was associated with shorter EFS. IGHV mutation status had an impact on EFS with pcb (U-IGHV vs. M-IGHV, HR 4.44, 95%CI 2.88-6.85, p<0.001) but not with ibr (HR 1.52, 95%CI 0.81-2.85, p=0.19).
In the pcb arm, mutations in NOTCH1 (HR 2.26, 95%CI 1.35-3.76, p<0.01), NRAS/KRAS/BRAF, (HR 5.38, 95%CI 1.92-15.03, p<0.01) and NFKBIE (HR 4.12, 95%CI 1.97-8.64, p<0.001) but not TP53 (HR 1.24, 95%CI 0.57-2.68, p=0.59) were significantly associated with a shorter EFS. In contrast, with ibr treatment, only TP53 (HR 2.88, 95%CI 1.31-6.32, p<0.01) and NFKBIE (HR 2.45, 95%CI 1.03-5.87, p=0.04) were associated with shorter EFS. Notably, in the full trial population (i.e. including the w&w cohort) TP53 mutations were associated with shorter EFS only when combined with U-IGHV (HR 2.37, 95%CI 1.22-4.63, p<0.05) but not with M-IGHV (HR 0.53, 95%CI 0.26-1.10. p=0.09).
In the w&w cohort high risk markers were rare and only +(12) (compared to del(13q)(HR 4.66, 95%CI 1.4-15.51, p<0.05) and mutations in TP53 (HR 4.88, 95%CI 1.49-16.04, p<0.01), SF3B1 (HR 5.01, 95%CI 1.53-16.45, p<0.01), BIRC3 (HR 3.43, 95%CI 1.05-11.23, p<0.05) and NFKBIE (HR 12.58, 95%CI 3.68-42.99, p<0.001) were significantly associated with shorter EFS.
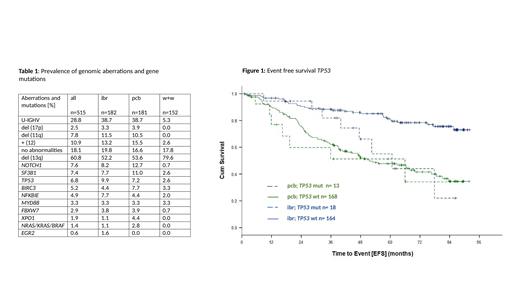
Significant EFS benefit from ibr as compared to pcb with HR<0.2 was observed in subgroups with U-IGHV (HR 0.18, 95%CI 0.1-0.3, p<0.001); del(11q) (HR 0.10, 95%CI 0.04-0.29, p<0.001) and cases with mutations in NOTCH1 (HR 0.18, 95%CI 0.06-0.55, p<0.01) and NFKBIE (HR 0.15, 95%CI 0.04-0.53, p<0.01), while no significant benefit was observed with del(17p) (HR 0.68, 95%CI 0.16-2.85, p=0.59) and mutated TP53 (HR 0.69, 95%CI 0.25-1.92, p=0.48, figure 1). Regarding OS none of the subgroups defined by genetic markers derived significant benefit from ibr as compared to pcb.
In multivariable analysis for EFS including treatment arms, clinical disease characteristics and genetic markers as variables, ibr treatment was an independent favorable factor (HR 0.25, 95%CI 0.16-0.37, p<0.001), while U-IGHV (HR 3.38, 95%CI 2.29-4.98 p<0.001), del(17p) (HR 3.53, 95%CI 1.76-7.06, p<0.001), +(12) (HR 1.58, 95%CI 1.01-2.45 p<0.05) and mutated NFKBIE (HR 2.22, 95%CI 1.23-4.01, p<0.01) were independent adverse prognostic factors.
Conclusion: In addition to the well-known prognostic factors, also mutated SF3B1, NFKBIE and BIRC3 are associated with shorter EFS in low risk early stage CLL. While some genetic subgroups derived particular EFS benefit from ibr, patients with high-risk TP53 aberration and/or del(17p) did not. In summary, the CLL12 trial fails to demonstrate survival superiority by early ibr treatment, including genetic subgroups and w&w remains the standard of care for all patients with Binet stage A CLL.
Disclosures
Tausch:Abbvie: Consultancy, Honoraria, Other: Travel Support, Research Funding, Speakers Bureau; Roche: Consultancy, Honoraria, Research Funding, Speakers Bureau; Janssen-Cilag: Consultancy, Honoraria, Other: travel support, Speakers Bureau; AstraZeneca: Consultancy, Honoraria, Other: travel support, Speakers Bureau; BeiGene: Consultancy, Other: Travel support, Speakers Bureau. Yosifov:Bayer: Current equity holder in publicly-traded company; Roche: Current equity holder in publicly-traded company. Schneider:BeiGene: Other: travel support; AstraZeneca: Consultancy, Honoraria, Speakers Bureau; Abbvie: Honoraria, Speakers Bureau; Jannsen Cilag: Consultancy. Fink:AstraZeneca: Consultancy, Honoraria, Research Funding; Abbvie: Other: travel support. Eichhorst:AstraZeneca: Consultancy, Honoraria, Research Funding, Speakers Bureau; BeiGene: Consultancy, Honoraria, Research Funding, Speakers Bureau; Gilead: Consultancy, Research Funding; Janssen: Consultancy, Research Funding, Speakers Bureau; Lilly: Consultancy, Speakers Bureau; MSD: Consultancy, Honoraria, Speakers Bureau; F. Hoffmann-La Roche Ltd: Honoraria, Research Funding, Speakers Bureau; Abbvie: Consultancy, Honoraria, Research Funding, Speakers Bureau. Fischer:Abbvie: Honoraria, Other: TRavel support; AstraZeneca: Consultancy; Roche: Honoraria, Other: Travel Support. Hallek:Abbvie: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Honoraria, Research Funding; BeiGene: Consultancy, Honoraria, Research Funding; Gilead: Consultancy, Honoraria, Research Funding; AstraZeneca: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding. Langerbeins:Abbvie: Honoraria, Other: travel support; Janssen: Honoraria, Other: travel support, Research Funding; Beigene: Honoraria, Other: travel support; AstraZeneca: Honoraria, Other: travel support. Stilgenbauer:Amgen: Consultancy, Honoraria, Other: travel support, Research Funding; Abbvie: Consultancy, Honoraria, Other: travel support, Research Funding; Novartis: Consultancy, Honoraria, Other: travel support, Research Funding; Janssen: Consultancy, Honoraria, Other: travel support, Research Funding; Sunesis: Consultancy, Honoraria, Other: travel support, Research Funding; Gilead: Consultancy, Honoraria, Other: travel support, Research Funding; Celgene: Consultancy, Honoraria, Other: travel support, Research Funding; AstraZeneca: Consultancy, Honoraria, Other: travel support, Research Funding; Roche: Consultancy, Honoraria, Other: travel support, Research Funding; GSK: Consultancy, Honoraria, Other: travel support, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal